Dosage compensation in Drosophila.
Dosage compensation is the process that ensures equal levels of
X-linked gene products in males and females in species in which the sexes
differ in the number of X chromosomes they possess. In Drosophila,
dosage compensation is achieved by doubling the transcript levels of X-linked
genes in males. During the past 10 years there has been enormous progress
in understanding both how dosage compensation is controlled in flies so
that it happens only in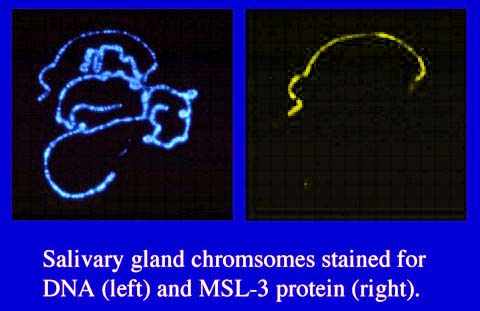 males, as well as the manner in which it works to modulate the level of
transcription of the X chromosome. Dosage compensation in flies is
mediated by 6 protein coding genes [maleless (mle), male-specific lethal-1(msl-1),
male-specific lethal-2 (msl-2), male-specific lethal-3 (msl-3), male (?)
absent on the first (mof), and JIL-1 kinase], collectively referred to
here as the msl genes, together with the non-coding RNA products of two
additional genes, roX1 and roX2 (roX = RNA on the X).
males, as well as the manner in which it works to modulate the level of
transcription of the X chromosome. Dosage compensation in flies is
mediated by 6 protein coding genes [maleless (mle), male-specific lethal-1(msl-1),
male-specific lethal-2 (msl-2), male-specific lethal-3 (msl-3), male (?)
absent on the first (mof), and JIL-1 kinase], collectively referred to
here as the msl genes, together with the non-coding RNA products of two
additional genes, roX1 and roX2 (roX = RNA on the X).
There is substantial evidence that the products of all of these genes
function together in a complex, termed the compensasome, to mediate dosage
compensation by altering the chromatin structure of the X chromosome in
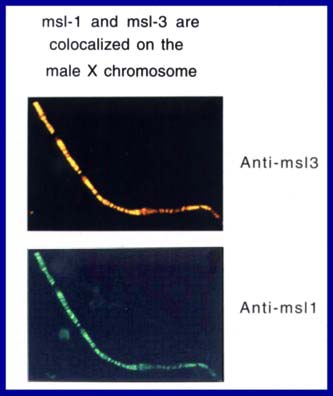
males. The products of these genes are all specifically associated
with the same set of hundreds of sites along the male X chromosome Figures
1, 2), and all of the MSL proteins and at least one of the ROX RNAs must
be present for the association of the compensasome with these sites. The
requirement that all the MSL proteins be present for the compensasome to
form allows dosage compensation to be made male-specific by preventing
the production of just one component in females. Indeed, translation
of msl-2 transcripts is repressed by the protein product of the female-specific,
master sex determination gene, Sex-lethal (Sxl). Therefore, in females,
MSL-2 protein is not generated, so active compensasomes do not form.
The likely mechanism by which the compensasome alters chromatin structure
(thereby leading to hypertranscription) is via the modification of histones.
An isoform of histone H4 acetylated at lysine 16, H4Ac16, is enriched at
a set of sites on the male X chromosome whose locations correlate with
the sites where compensasomes are found. The mof gene encodes a histone
acetyltransferase, and a partially purified complex containing MOF is able
to acetylate histone H4 specifically at lysine 16, as is recombinant MOF
alone. That a second histone modification may be involved in dosage
compensation has been suggested by the finding that JIL-1 kinase is associated
with the compensasome, and phosphorylates histone H3 at ser10. Thus
modifications of histones are a fundamental part of dosage compensation,
as they are of transcriptional regulation more generally.
One focus of our current research on dosage compensation is on understanding
how the distribution of compensasomes along the X chromosome is achieved.
Almost everything known about the distribution of compensasomes along the
male X comes from examination of polytene salivary chromosomes. The
relevant facts are as follows. In wild type there are several hundred sites
at which compensasomes are found along the X chromosome. These sites
are reproducible both between cells and between organisms. Although the
places where compensasomes are found along the X chromosome are referred
to by all of us in the field as ‘sites’, they are in fact not points,
but rather 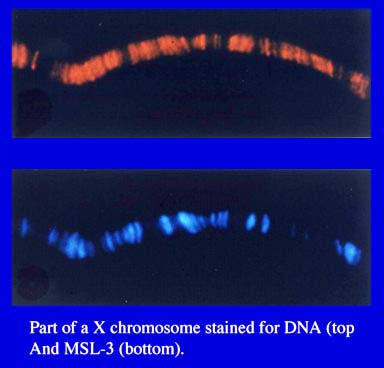 bands
(small segments of chromosome) that roughly span the size range of salivary
chromosome bands seen with DNA stains (i.e. a few 10’s to several 100’s
of kb in length) (Figure 3). The compensasome bands do not correspond
to the bands where DNA is condensed. An understanding of the distribution
of compensasomes along the X chromosome needs to encompass not only how
compensasomes get to these several hundred sites, but also how the ends
of each compensasome band are delimited. To address these goals we
are using both molecular and cytogenetic approaches to dissect the processes
that govern the distribution of compensasomes along the male X chromosome
bands
(small segments of chromosome) that roughly span the size range of salivary
chromosome bands seen with DNA stains (i.e. a few 10’s to several 100’s
of kb in length) (Figure 3). The compensasome bands do not correspond
to the bands where DNA is condensed. An understanding of the distribution
of compensasomes along the X chromosome needs to encompass not only how
compensasomes get to these several hundred sites, but also how the ends
of each compensasome band are delimited. To address these goals we
are using both molecular and cytogenetic approaches to dissect the processes
that govern the distribution of compensasomes along the male X chromosome
Publications on dosage compensation
Original research publications.
Kuroda, M. I., Kernan, M. J., Kreber, R., Ganetzky, B. and Baker, B. S.
(1991). The maleless protein associates with the X chromosome to
regulate dosage compensation in Drosophila. Cell 66: 935-948.
Kernan, M. J., Kuroda, M. I., Kreber, R., Baker, B. S., and Ganetzky,
B. (1991). napts, a mutation affecting sodium channel activity in
Drosophila, is an allele of mle, a regulator of X chromosome transcription.
Cell 66: 949-961.
Gorman, M., Kuroda, M. and Baker, B.S. (1993). Regulation of the sex-specific
binding of the maleless dosage compensation protein to the male X chromosome
in Drosophila. Cell, 72: 39-49.
Gorman, M., Franke,
A., and Baker, B. S., (1995) Molecular characterization of the male-specific
lethal-3 gene and investigations of the regulation of dosage compensation
in Drosophila. Development, 121: 463-475.
Bashaw, G. J., and Baker,
B. S. (1995). The msl-2 dosage compensation gene of Drosophila encodes
a putative DNA-binding protein whose expression is sex-specifically regulated
by Sex-lethal. Development, 121: 3245-3258.
Franke, A., Dernberg,
A., Bashaw, G. J., and Baker, B. S. (1996). Evidence that MSL mediated
dosage compensation in Drosophila begins at blastoderm. Development
122: 2751-2760.
Marín, I., Franke, A., Bashaw, G. J., and Baker, B. S. (1996).
Dosage compensation in flies: a regulatory system adapting to chromosome
evolution.. Nature, 383: 160-163.
Bashaw, G. J., and Baker, B. S., (1997) The regulation of the Drosophila
msl-2 gene reveals a function for Sex-lethal in translational control.
Cell, 89: 789-798.
Franke, A. and Baker, B. S., (1999) The rox1 and rox2 RNAs are
essential components of the Compensasome, which mediates dosage compensation
in Drosophila. Molec. Cell 4: 117-122.
Marin, I., and Baker, B. S. (2000). Origin and evolution of the
regulatory gene male-specific lethal 3. Mol. Biol. Evol.17: 1240-1250.
Reviews.
Gorman, M., and Baker, B. S. (1994) How flies make one equal two:
dosage compensation in Drosophila. TIGS 10: 376-380.
Baker, B. S., Marin, I., and Gorman, M. (1994) Dosage compensation in
Drosophila. Annu. Rev. Genetics, 28: 491-521.
Bashaw, G. J., and Baker, B. S. (1996). Dosage Compensation and
chromatin structure in Drosophila. Curr. Opinions in Genet. and Dev.
496-501.
Franke, A. and Baker, B. S., (2000). Dosage compensation rox!
Curr. Opinion in Cell Biol. 12:351-354.
Marin, I., Siegal,
M. L. and Baker, B. S., (2000). The evolution of dosage compensation
mechanisms. BioEssays, 22: 1106-1114.
 males, as well as the manner in which it works to modulate the level of
transcription of the X chromosome. Dosage compensation in flies is
mediated by 6 protein coding genes [maleless (mle), male-specific lethal-1(msl-1),
male-specific lethal-2 (msl-2), male-specific lethal-3 (msl-3), male (?)
absent on the first (mof), and JIL-1 kinase], collectively referred to
here as the msl genes, together with the non-coding RNA products of two
additional genes, roX1 and roX2 (roX = RNA on the X).
males, as well as the manner in which it works to modulate the level of
transcription of the X chromosome. Dosage compensation in flies is
mediated by 6 protein coding genes [maleless (mle), male-specific lethal-1(msl-1),
male-specific lethal-2 (msl-2), male-specific lethal-3 (msl-3), male (?)
absent on the first (mof), and JIL-1 kinase], collectively referred to
here as the msl genes, together with the non-coding RNA products of two
additional genes, roX1 and roX2 (roX = RNA on the X).

 bands
(small segments of chromosome) that roughly span the size range of salivary
chromosome bands seen with DNA stains (i.e. a few 10’s to several 100’s
of kb in length) (Figure 3). The compensasome bands do not correspond
to the bands where DNA is condensed. An understanding of the distribution
of compensasomes along the X chromosome needs to encompass not only how
compensasomes get to these several hundred sites, but also how the ends
of each compensasome band are delimited. To address these goals we
are using both molecular and cytogenetic approaches to dissect the processes
that govern the distribution of compensasomes along the male X chromosome
bands
(small segments of chromosome) that roughly span the size range of salivary
chromosome bands seen with DNA stains (i.e. a few 10’s to several 100’s
of kb in length) (Figure 3). The compensasome bands do not correspond
to the bands where DNA is condensed. An understanding of the distribution
of compensasomes along the X chromosome needs to encompass not only how
compensasomes get to these several hundred sites, but also how the ends
of each compensasome band are delimited. To address these goals we
are using both molecular and cytogenetic approaches to dissect the processes
that govern the distribution of compensasomes along the male X chromosome